Institute for Quantum Life Science
DNA Damage Chemistry Research Group
Group Leader: Akamatsu, K.
Members: Moribayashi, K.
Nakano, T.
Ishii, C.
Mechanisms of Mutagenesis Group
Group Leader: Shikazono, N.
Kayamura, S.
Research Activities
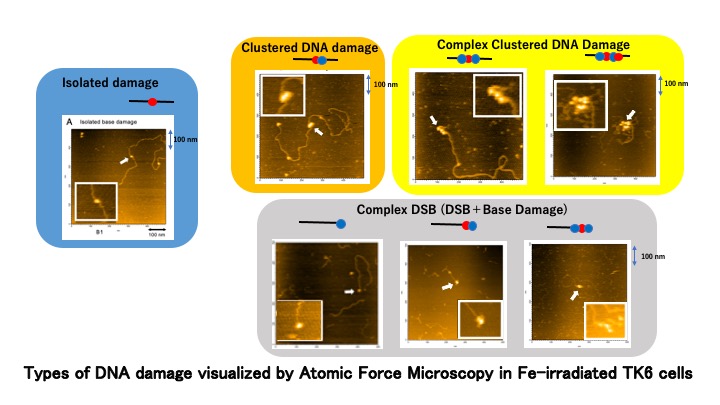
The main goal of the DNA Damage Chemistry Research Group is to clarify the nature of DNA damage induced by various agents, especially damage from ionizing radiation. The group aims to utilize new experimental techniques that can unveil the structure of DNA damage as well as the recognition of DNA damage by repair enzymes at nanometer scales. The focus of the group is currently on “clustered DNA damage”, in which two or more DNA lesions are located within one to two helical turns of DNA (within several nanometers along the DNA). Clustered DNA damage is considered to be challenging to repair, and thus, a critical type of damage induced by ionizing radiation. However, its presence and microstructure has remained elusive, as few experimental methods were able to obtain data on the spatial distribution of DNA lesions. This research group has established a novel approach for measuring the level of localization of DNA damage by directly visualizing the damage. Atomic force microscopy (AFM) has a resolution at the nanometer/subnanometer scale, and thus DNA can be directly visualized. The research group has labeled DNA damage (abasic sites) by attaching aldehyde reactive probes with biotin, and then attaching streptavidin to the biotin. The large molecular size enables the detection of streptavidin by AFM. Abasic sites were directly induced, or further revealed by removing the damaged base by DNA glycosylases, after irradiation. The complex nature of the clustered DNA damage was visualized for the first time by this method. The research group has further developed a promising method to detect clustered DNA damage, using fluorescence resonance energy transfer (FRET). In this method, aldehyde/ketone moieties such as at abasic sites in irradiated DNA are labeled by aminooxyl fluorophores. Fluorescence anisotropy enabled the estimation of the apparent base-pair separations between lesions in a cluster produced by an ion track, and revealed that the yield of clustered abasic sites increased with increasing linear energy transfer (LET) of the radiation. Both the AFM and FRET approach confirmed that these novel analyses have the potential to be used to discover the qualitative and the quantitative differences of clustered DNA damage produced by various types of ionizing radiation.
The goal of the Mechanism of Mutagenesis Group is to elucidate the underlying mechanisms of the induction of mutations, which are highly relevant to carcinogenesis and the evolution of life. One aspect of the research of this group focuses on the events at the very early stages (around a femtosecond to picosecond) within a space in the scale of nanometers after energy transfer from ionizing radiation, especially from ion particles. Using Monte-Carlo simulations, the research group has demonstrated that, when water is exposed to densely ionizing ion particles, some of the secondary electrons ejected from water molecules are trapped within the electric potential created by the ionized water molecules. This result led to the realization that the radial dose near the track of a densely ionizing ion particle is much higher than previously understood. This highly localized energy deposition is considered to produce a high yield of clustered DNA damage, and thus have important implications for the drastic effect of ion particles on cells.
Publications
- A model for analysis of the yield and the level of clustering of radiation-induced DNA-strand breaks in hydrated plasmids, N. Shikazono, A. Yokoya, A. Urushibara, M. Noguchi and K. Fujii, Radiat. Prot. Dosimetry, 143, 181-185 (2011).
- Incorporation of the effect of the composite electric fields of molecular ions as a simulation tool for biological damage due to heavy-ion irradiation, K. Moribayashi, Phys. Rev. A, 84, 012702-1 – 012702-7 (2011).
- Photo-electron spectra produced from the irradiation of X-ray free electron laser light pulses onto non-spherically symmetric targets. K. Moribayashi, Phys. Scr., T144, 014049-1 – 014049-3 (2011).
- Radiation damage of C1H1, N1H1 and O1H1 clusters induced by irradiation with X-ray free electron lasers, T.Kai, Phys. Scr., T144, 014050-1 – 014050-3 (2011).
- Determination of Structural Parameters of Protein-Containing Reverse Micellar Solution Based on Near-Infrared Absorption Spectroscopy, H. Murakami, T. Nishi and Y. Toyota, J. Phys. Chem. B, 115, 5877-5885 (2011).
- A novel technique using DNA denaturation to detect multiply induced single-strand breaks in a hydrated plasmid DNA molecule by X-ray and 4He2+ ion irradiation. A. Yokoya, N. Shikazono, K. Fujii, M. Noguchi and A. Urushibara, Radiat. Prot. Dosimetry, 143, 219-225 (2011).
- Monte Carlo code for the damage of bio-molecules irradiated by X-Ray free electron lasers: Incorporation of electron impact ionization processes, K. Moribayashi, Progress in Nuclear Science and Technology (PNST), 2, 893 - 897 (2011).
- Observation of organelles in Leydig cells by contact soft X-ray microscopy with a laser plasma X-ray source, M. Kado, M. Ishino, S. Tamotsu, K. Yasuda, M. Kishimoto, M. Nishikino, Y. Kinjo and K. Shinohara, AIP conference proceedings, 1365, 391-394 (2011).
- Determination of structural parameters of protein-containing reverse micellar solution by near-infrared absorption spectroscopy, H. Murakami, T. Nishi and Y. Toyota, J. Phys. Chem. B, 115, 5877-5885 (2011).
- Series: Physics of atomic, molecular and optics due to short wavelength FEL – the role of atomic and molecular physics for the analysis for the structure of bio-molecules, K. Moribayashi, Syototsu, The Atomic Collision Society of Japan, 9, 8-13 (2012) (in Japanese) (Review).
- Observation of organelle by a laser plasma X-ray microscope, M. Kado, M. Kishimoto, M. Ishino, S. Tamotsu, K. Yasuda and K. Shinohara, AIP conference proceedings, 1465, 246-250 (2012).
- Development of single shot soft X-ray contact microscopy system for nano-scale dynamics measurement of living biological specimen, M. Kishimoto, M. Kado, M. Ishino, S. Tamotsu, K. Yasuda and K. Shinohara, AIP conference proceedings, 1465, 45-47 (2012).
- Incorporation of the effect of the composite electric fields of molecular ions as a simulation tool for biological damage due to heavy ion irradiation II, K. Moribayashi, AIP conference proceedings, 1465, 241-245 (2012).
- Terahertz absorption spectroscopy of protein-containing reverse micellar solution, H. Murakami, Y. Toyota, T. Nishi and S. Nashima, Chem. Phys. Lett., 519-520, 105-109 (2012).
- The mutagenic potential of 8-oxoG/single strand break-containing clusters depends on their relative positions. M. Noguchi, A. Urushibara, A. Yokoya, P. O’Neill and N. Shikazono, Mutat. Res., 732, 34-42 (2012).
- Yield of single- and double-strand breaks and nucleobase lesions in fully hydrated plasmid DNA films irradiated with high-LET charged particles. T. Ushigome, N. Shikazono, K. Fujii, R. Watanabe, M. Suzuki, C. Tsuruoka, H. Tauchi and A. Yokoya, Radiat. Res., 177, 614-627 (2012).
- Generation of strongly coupled Xe cluster nanoplasmas by low intensive soft X-ray laser irradiation, S. Namba, N. Hasegawa, M. Kishimoto, M. Nishikino and T. Kawachi, AIP conference proceedings, 1465, 69-73 (2012).
- Electron spectra of xenon clusters irradiated with a laser-driven plasma soft x-ray laser pulse, S. Namba, N. Hasegawa, M. Kishimoto, M. Nishikino, K. Takiyama and T. Kawachi, Physical Rev. A, 84, 53202 (2012).
- Movement of secondary electrons due to the irradiation of heavy ions: Role of the composite electric field formed from the polarization of molecules and molecular ions, K. Moribayashi, Rad. Phys. Chem., 85, 36 - 41 (2013).
- Demonstrations for the effect of composite electric fields of molecular ions on the motion of secondary electrons due to ion irradiation, K. Moribayashi, Nucl. Instr. Methods Phys. Res. B, 314, 30 - 33 (2013).
- In situ observation of cellular organelles with a contact x-ray microscope, M. Kado, M. Kishimoto, S. Tamotsu, K. Yasuda, and K. Shinohara, J. Physics: Conf. Ser. 463, 012056-1-4 (2013).
- Significance of DNA polymerase I in in vivo processing of clustered DNA damage, N. Shikazono, K. Akamatsu, M. Takahashi, M. Noguchi, A. Urishibara, P. O’Neill, A. Yokoya, Mutat. Res., 749,9-15 (2013)
- Development of ‘leaky’ liposome triggered by radiation applicable to a drug reservoir and a simple radiation dosimeter, K. Akamatsu, Appl. Radiat. Isotop., 74, 144-151 (2013).
- A mehodology for estimating localization of apurinic/apyrimidinic sites in DNA using fluorescence resonance energy transfer, K. Akamatsu and N. Shikazono, Anal. Biochem., 433, 171-180 (2013).
- Nanometer-scale water droplet free from the constraint of reverse micelles at low temperatures, H. Murakami, T. Sada, M. Yamada and M. Harada, Physical. Rev. E, 88, 052304 (2013).
- Relaxation of plasma created from irradiation of a heavy Ion, K. Moribayashi, JPS Conference Proceedings, 1, 013089 (2014).
- Radial dose calculation due to the irradiation of a heavy ion: Role of composite electric field formed from the polarization of molecules and molecular ions, K. Moribayashi, Rad. Phys. Chem., 96, 211 - 216 (2014).
- Radial dose for heavy particle beam, K. Moribayashi, The Atomic Collision Society of Japan, 11, 73 - 91 (2014) (in Japanese) (Review).
- Towards Laser Driven Hadron Cancer Radiotherapy: A Review of Progress, K.W.D. Ledingham, P.R. Bolton, N. Shikazono and C.-M. Charlie Ma, Appl. Sci., 4, 402-433 (2014)(Review).
- Localization estimation of ionizing radiation-induced abasic sites in DNA in the solid state using fluorescence resonance energy transfer, K. Akamatsu, N. Shikazono, and T. Saito, Radiat. Res., 183, 105-113 (2015).
- Chemical repair activity of free radical scavenger, edaravone: Reduction reactions with dGMP hydroxyl radical adducts and suppression of base lesions and AP sites on irradiated plasmid DNA, K.Hata, A. Urushibara, S. Yamashita, M. Lin, Y. Muroya, N. Shikazono, A. Yokoya, H. Fu and Y. Katsumura, J. Radiat. Res., 56, 59-66 (2015).
- Simulation study of radial dose due to the irradiation of a swift heavy ion aiming to advance the treatment planning system for heavy particle cancer therapy: the effect of emission angles of secondary electrons, K. Moribayashi, Nucl. Instru. Methods Phys. Res. B, 365 592 – 595 (2015).
- Development of the radial dose distribution function relevant to the treatment planning system for heavy particle cancer therapy, K. Moribayashi, Phys. Scr. 90 054013 (2015).
- Effect of recombination between a molecular ion and an electron on radial dose in the irradiation of a heavy ion, K. Moribayashi, Applied Physics Research, 8, 138 – 148 (2016).
- Efficiency of radiation-induced base lesion excision and the order of enzymatic treatment. Shiraishi I, Shikazono N, Suzuki M, Fujii K, Yokoya A. Int J Radiat Biol. 93:295-302 (2017).
- Radiation-induced clustered DNA lesions: repair and mutagenesis. Sage, E. and Shikazono, N. Free Rad. Med. Biol. 107:125-135 (2017)
- Effect of track potentials on the movement of secondary electrons due to irradiation of heavy ions, Moribayashi, K., J. Phys. Soc. Jpn., 86:024301-1 - 024301-5 (2017)
- Simple formulas for heavy-ion-irradiation-induced electric field, Moribayashi, K., Nucl. Instr. Methods Phys. Res. B. 408: 241-243 (2017)
- New method for estimating clustering of DNA lesions induced by physical/chemical mutagens using fluorescence anisotropy. Akamatsu K, Shikazono N, Saito T. Anal Biochem. 536:78-89 (2017).
- Proposal for Experiment Systems Using Laser-Driven Heavy Ions and XFELs to Understand Physical Phenomena Occurring near the Incident Ion Path. Moribayashi, K., Proc. 15th Int. Conf. X-ray, 202:121-123 (2018).
- Effect of the track potential on the motion and energy flow of secondary electrons created from heavy-ion irradiation, Moribayashi, K., Radiat. Phys. Chem. 146: 68-72 (2018).
- Mutagenic potential of 8-oxo-7,8-dihydroguanine (8-oxoG) is influenced by nearby clustered lesions. Shikazono, N.* and Akamatsu, K. Mutation Research - Fundamental and Molecular Mechanisms of Mutagenesis, 810: 6-12 (2018).
- Application of simple formulas to track potential in heavy-ion-beam simulation, Moribayashi, K., Trans. Material Res. Soc. Japan, 43: 267-270 (2018).
- Heat diffusion in heavy-ion beam irradiation, Moribayashi, K., Xray Spectrometry, 48:708-711 (2019).
- Tyrosyl-DNA phosphodiesterase 2 (TDP2) repairs topoisomerase 1 DNA-protein crosslinks and 3ʹ-blocking lesions in the absence of tyrosyl-DNA phosphodiesterase 1 (TDP1). Tsuda, M., Kitamatsu, K., Kumagai, C., Sugiyama, K., Nakano, T. Ide, H. DNA repair 91-92: 102849 (202).
- A Simplified Cluster Analysis of Electron Track Structure for Estimating Complex DNA Damage Yields. Matsuya, Y,. Nakano, T., Kai, T., Shikazono, N., Akamatsu, K., Yoshii, Y., Sato, T. Int J Mol Sci. 21: pii: E1701 (2020).
- TDP1-dependent repair of formaldehyde-induced DNA-protein cross-links in chicken DT40 cells. Nakano, T., Soulkamy, M.I., Tuda, M., Sasanuma, H., Takata, M., Masunaga, S., Takeda, S., Ide, H., Bessho, T., PLoS One, 15: e0234859
- Strand with mutagenic lesion is preferentially used as a template in the region of a bi-stranded clustered DNA damage site in Escherichia coli. Shikazono, N.* and Akamatsu, K., Sci. Rep., 10, 9737 (2020).
- Observation of water-window soft x-ray emission from laser-produced Au plasma under optically thin condition. John, C., Kishimoto, M., Matsumoto, Y., Morishita, T., Higashiguchi, T., Endo, T., Sunahara, A., Johzaki, T., Namba, S., High Energy Density Phys. 37: 100845 (2020)
- Characterization of gamma irradiation-induced mutations in Arabidopsis mutants deficient in non-homologous end joining. Du, Y., Yoshihiro Hase, Y., Satoh, K., Shikazono, N., J. Radiat. Res., 61(5):639-647 (2020).
- Application of atomic and molecular data for plasma production and cancer therapy by heavy particle irradiation。Moribayashi, K. Japan. J. Appl. Phys. 59, SH0801 (2020).
- Fluorescence anisotropy study of radiation-induced DNA damage clustering based on FRET. Akamatsu, K., Shikazono, N., Saito, T. Anal. Bioanal. Chem., 413:1185-1192 (2021).
- Debye shield formed by track potential and transport of secondary electrons in heavy ion irradiation. Moribayashi, K., Radiat. Phys. Chem. 184: 109436 (2021).
- Proton-Cluster-Beam Lethality and Mutagenicity in Bacillus subtilis Spores. Hase, Y., Sato, K., Chiba, A., Hirano, Y., Moribayashi, K., Narumi, K., Quantum Beam Sci. 5: 25 (2021).
- Formation of clustered DNA damage in vivo upon irradiation with ionizing radiation: visualization and analysis with atomic force microscopy. Nakano, T., Akamatsu, K., Tsuda, M., Tujimoto, A., Hirayama, R., Hiromoto, T., Tamada, T., Ide, H., Shikazono, N. Proceedings of the National Academy of Sciences, USA. 119(13): e2119132119 (2022)
