Joint Release by Hokkaido University and the Japan Agency for Medical Research and Development (AMED).
Adenosine triphosphate (ATP) secreted from sensory neuron-interneuron crosstalk is key to the spreading of inflammation across joints, acting as a neurotransmitter and inflammation enhancer.
Rheumatoid Arthritis (CC BY-NC 2.0 Leides Moura).
Rheumatoid arthritis is a chronic inflammatory autoimmune disorder that primarily affects joints. One of the key features of this disease is remote inflammation, where inflammation spreads from one joint to another. Research has shown that neural circuits or cells migrated from the joints are involved in inflammation spread, but the detailed mechanism by which this occurs has not been elucidated.
A team of researchers from Japan and the USA, led by Professor Masaaki Murakami at Hokkaido University, have revealed that remote inflammation spreads by neuron crosstalk, and that adenosine triphosphate (ATP) plays a key role in this process. Their findings, published in the Journal of Experimental Medicine, may lead to new therapies and treatments for inflammatory diseases.
Inflammation is a part of the natural immune response that occurs in response to infection or irritation. It is a process by which the immune system, involving immune cells, blood vessels, and molecular mediators, attempts to clear out the pathogens and damaged cells and thereafter repair the damage. However, excessive inflammation is a disorder in itself, and is seen in diseases such as hay fever, atherosclerosis, psoriasis, and rheumatoid arthritis, among others.
In this study, the authors used previous observations of the gateway reflex—an immune response mechanism whereby specific neural signals change the state of specific blood vessels to allow immune cells to enter tissue, leading to local inflammation—to hypothesize that neural crosstalk could be responsible for remote inflammation.
They tested this hypothesis through experiments in rheumatoid arthritis models in mice. The mice were divided into control and test groups. In the test groups, the sensory neural circuits between the left and right ankle joints were interrupted. Arthritis of the left ankle was then induced in both sets of mice and the spread of arthritis to the right ankle was observed.
Their results showed that the inflammation signal in one joint is transmitted to the other via a sensory neuron connection through the spinal cord, leading to inflammation in both joints. Specifically, inflammation in one joint led to an increase of ATP in both joints, which triggered an increase of a signal molecule that resulted in inflammation. Blocking this pathway prevented the spread of inflammation.
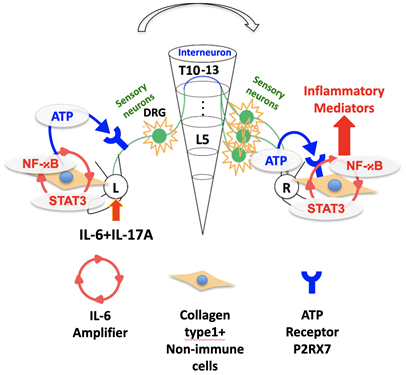
The inflammation signal (IL-6 amplifier) in the affected joint (L) causes the secretion of ATP that transmits a signal through the spinal cord (DRG, L5), which in turn triggers an increase in ATP concentrations in the unaffected joint (R) that results in inflammation in that joint (Rie Hasebe, et al. Journal of Experimental Medicine. May 10, 2022).
As this study was performed in mice models, it is necessary to determine if the findings apply to rheumatoid arthritis and other chronic inflammatory diseases in humans. If so, it could provide a therapeutic target for various diseases with spreading inflammation.
###
The researchers involved in this study include Rie Hasebe, Kaoru Murakami, Nada Halaka, Junko Nio-Kobayashi, Toshihiko Iwanaga, Masahiko Watanabe, Daisuke Kamimura, Yuki Tanaka, Masaaki Murakami at Hokkaido University. Rie Hasebe is also affiliated with the National Institutes of Natural Sciences and the Moonshot Research and Development Project, Japan Agency for Medical Research and Development (AMED); Yuki Tanaka is also affiliated with the National Institute for Quantum Science and Technology (QST) and the Moonshot Research and Development Project, Japan Agency for Medical Research and Development (AMED); and Masaaki Murakami is also affiliated with affiliated with the National Institutes of Natural Sciences, the National Institute for Quantum Science and Technology (QST), and the Moonshot Research and Development Project, Japan Agency for Medical Research and Development (AMED).

The Murakami group at Hokkaido University. Rie Hasebe (third row, right; white long-sleeved shirt holding a blue jacket), Kaoru Murakami (last row, left; dark violet scrubs), Yuki Tanaka (second row, center; gray zip-up hoodie), and Masaaki Murakami (first row, center; black zip-up hoodie) are key contributors to the paper. (Photo: Masaaki Murakami)
Original Article:
Rie Hasebe, et al. ATP spreads inflammation to other limbs through crosstalk between sensory neurons and interneurons. Journal of Experimental Medicine. May 10, 2022.
DOI: 10.1084/jem.20212019
Funding:
This work was supported by Japan Society for the Promotion of Science
(JSPS) KAKENHI; Ministry of Education, Culture, Sports, Science and Technology (MEXT) Quantum Leap Flagship Program (Q-LEAP; JPMXS0120330644); Japan Agency for Medical Research and Development (AMED); Takeda Science Foundation; Institute for Fermentation Osaka; Mitsubishi Foundation; Chugai Pharma Research Grant; and Akiyama Life Science Foundation. This study was also supported partly by the Grant for Joint Research Program of the Institute for Genetic Medicine, Hokkaido University, by the Photo-excitonix Project, Hokkaido University, and by the Promotion Project for Young Investigators at Hokkaido University.
