
 Our research aims to understand carcinogenesis and find ways to prevent cancer by applying quantum technologies, such as quantum measurement and sensing, as tools for cancer research.
Our research aims to understand carcinogenesis and find ways to prevent cancer by applying quantum technologies, such as quantum measurement and sensing, as tools for cancer research.
Cancer is a disease that will affect more than half of us at some point in our lives and is the leading cause of death. Although there have been innovations in cancer treatment based on the results of molecular biology, such as genomic drug discovery and immunotherapy, cancer incidence and mortality rates are increasing against the backdrop of an aging population. Therefore, by using quantum technology, we aim to understand cancer from a completely different perspective than before and lay the groundwork for further innovation in cancer treatment.

Team Leader, Tatsuhiko Imaoka
Research Topics
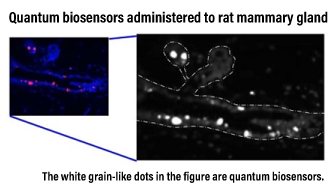 Until now, the application of quantum sensing using nanodiamond NV centers in biology has been limited to small experimental systems such as cultured cells and C. elegans. In order to apply the technology to mammals, which are more medically similar to humans, the following issues had to be addressed: (1) nanodiamond particles diffuse in the body, (2) the visible light necessary for quantum manipulation of NV centers cannot penetrate thick tissues, (3) physiological movements interfere with measurements at the microscopic scale, and (4) the toxicity of nanodiamonds is unknown. We solved these problems one by one by combining quantum sensing with advanced experimental animal techniques, localization and toxicity of injected nanodiamonds, and signal processing to mitigate the effects of biological motion, and finally succeeded in detecting elevated cellular temperature due to mastitis in rats. This achievement demonstrates the applicability of this technology to biomedical research using mammals and serves as a basis for expanding the potential applications of quantum sensing.
Until now, the application of quantum sensing using nanodiamond NV centers in biology has been limited to small experimental systems such as cultured cells and C. elegans. In order to apply the technology to mammals, which are more medically similar to humans, the following issues had to be addressed: (1) nanodiamond particles diffuse in the body, (2) the visible light necessary for quantum manipulation of NV centers cannot penetrate thick tissues, (3) physiological movements interfere with measurements at the microscopic scale, and (4) the toxicity of nanodiamonds is unknown. We solved these problems one by one by combining quantum sensing with advanced experimental animal techniques, localization and toxicity of injected nanodiamonds, and signal processing to mitigate the effects of biological motion, and finally succeeded in detecting elevated cellular temperature due to mastitis in rats. This achievement demonstrates the applicability of this technology to biomedical research using mammals and serves as a basis for expanding the potential applications of quantum sensing.
Recent Publications
FY2024
- Takahiro Hamoya, Kiichi Kaminaga, Ryuji Igarashi, Yukiko Nishimura, Hiromi Yanagihara, Takamitsu Morioka, Chihiro Suzuki, Hiroshi Abe, Takeshi Ohshima, Tatsuhiko Imaoka. Intravital Microscopic Thermometry of Rat Mammary Epithelium by Fluorescent Nanodiamond. Nanoscale Horizons 2024;9:1938-1947.
- Yuya Hattori , Kento Nagata , Ritsuko Watanabe , Akinari Yokoya , Tatsuhiko Imaoka. Super-competition as a Novel Mechanism of the Dose-rate Effect in Radiation Carcinogenesis: A Mathematical Model Study. Radiation Research 2025;203:61-72
- Nishimura Yukiko, Iizuka Daisuke, Takabatake Masaru, Daino Kazuhiro, Nishimura Mayumi, Morioka Takamitsu, Shimada Yoshiya, Kakinuma Shizuko, Imaoka Tatsuhiko. Characterization of molecular subtypes of rat mammary cancer and their association with environmental exposures. Anticancer Research 2024;44:4261-4272.
- Nagata Kento , Nishimura Mayumi , Daino Kazuhiro , Nishimura Yukiko , Hattori Yuya , Watanabe Ritsuko , Iizuka Daisuke , Yokoya Akinari , Suzuki Keiji , Kakinuma Shizuko , Imaoka Tatsuhiko. Luminal progenitor and mature cells are more susceptible than basal cells to radiation-induced DNA double-strand breaks in rat mammary tissue. Journal of Radiation Research 2024;65:640-650.
FY2023
- Suzuki Kenshi, Tsuruoka Chizuru, Morioka Takamitsu, Seo Hitomi, Ogawa Mari, Imaoka Tatsuhiko, Kakinuma Shizuko, Takahashi Akihisa. Combined effects of radiation and simulated microgravity on intestinal tumorigenesis in C3B6F1 ApcMin/+ mice. Life Sciences in Space Research 2024;41:202-209.
- Shang Yi, Morioka Takamitsu, Daino Kazuhiro, Nakayama Takafumi, Nishimura Mayumi, Kakinuma Shizuko. Ionizing radiation promotes, whereas calorie restriction suppresses, NASH and hepatocellular carcinoma in mice. International Journal of Cancer 2023;153:1529-2542
- Semba Ryoko, Morioka Takamitsu, Yanagihara Hiromi, Suzuki Kenshi, Tachibana Hirotaka, Hamoya Takahiro, Horimoto Yoshiya, Imaoka Tatsuhiko, Saito Mitsue, Kakinuma Shizuko, Arai Masami. Azithromycin induces read-through of the nonsense Apc allele and prevents intestinal tumorigenesis in C3B6F1 ApcMin/+ mice. Biomedicine & Pharmacotherapy , 164, 2023-06, DOI:https://doi.org/10.1016/j.biopha.2023.114968
- Hiromi Yanagihara, Takamitsu Morioka, Shunsuke Yamazaki, Yutaka Yamada, Hirotaka Tachibana, Kazuhiro Daino, Chizuru Tsuruoka, Yoshiko Amasaki, Mutsumi Kaminishi, Tatsuhiko Imaoka, Shizuko Kakinuma. Interstitial deletion of the Apc locus in β-catenin-overexpressing cells is a signature of radiation-induced intestinal tumors in C3B6F1 ApcMin/+ mice. Journal of radiation research, 64(3), 622 - 631, 2023-05
