Abstract
Objective Apathy is a common neuropsychological symptom in Alzheimer’s disease (AD), and previous studies demonstrated that neuronal loss and network disruption in some brain regions play pivotal roles in the pathogenesis of apathy. However, contributions of tau and amyloid-β (Aβ) depositions, pathological hallmarks of AD, to the manifestation of apathy remain elusive.
Methods Seventeen patients with AD underwent positron emission tomography (PET) with 11C-pyridinyl-butadienyl-benzothiazole 3 (11C-PBB3) and 11C-Pittsburgh compound-B (11C-PiB) to estimate tau and Aβ accumulations using standardised uptake value ratio (SUVR) images. 11C-PBB3 and 11C-PiB SUVR were compared between AD patients with high and low Apathy Scale (AS) scores. Additionally, volumetric and diffusion tensor MRI was performed in those areas where any significant difference was observed in PET analyses. Correlation and path analyses among AS and estimated imaging parameters were also conducted.
Results AD patients with high AS scores showed higher 11C-PBB3 SUVR in the orbitofrontal cortex (OFC) than those with low AS scores, while 11C-PiB SUVR in any brain regions did not differ between them. Elevated 11C-PBB3 SUVR in OFC, decreased OFC thickness and decreased fractional anisotropy (FA) in the uncinate fasciculus (UNC), which is structurally connected to OFC, correlated significantly with increased scores of the AS. Path analysis indicated that increased 11C-PBB3 SUVR in OFC affects apathy directly and through reduction of OFC thickness and subsequent decrease of FA in UNC.
Conclusions The present findings suggested that tau pathology in OFC may provoke focal neurotoxicity in OFC and the following disruption of the OFC-UNC network, leading to the emergence and progression of apathy in AD.
Image
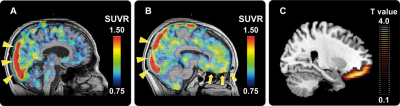
Representative11 C-PBB3 SUVR PET images of AD patients with low (A) and high (B) AS scores. High radioligand retention in the superior sagittal sinus (yellow triangles in panels a and b) is a non-specific radioactivity accumulation. AD patient with high AS score showed remarkably increased radioligand retention in the vicinity of OFC (yellow arrows in panel b) relative to the patient with low AS score (A). Statistical parametric map illustrates greater accumulations of PBB3-detectable tau lesions in OFC in AD patients with high AS scores relative to those with low AS scores (C). Data are thresholded at false discovery rate-corrected p value <0.05 and extent threshold >600 voxels (C).
PBB3, pyridinyl-butadienyl-benzothiazole 3; AD, Alzheimer’s disease;AS, Apathy Scale; OFC, orbitofrontal cortex; PET, positron emission tomography; SUVR, standardized uptake value ratio.
Reference
Tau-induced focal neurotoxicity and network disruption related to apathy in Alzheimer’s disease
Soichiro Kitamura1,2, Hitoshi Shimada1,※,Fumitoshi Niwa3, Hironobu Endo4, Hitoshi Shinotoh1,5, Keisuke Takahata1, Manabu Kubota1, Yuhei Takado1, Shigeki Hirano6, Yasuyuki Kimura1,7, Ming-Rong Zhang8, Satoshi Kuwabara6, Tetsuya Suhara1, Makoto Higuchi1
- Department of Functional Brain Imaging Research, National Institute of Radiological Sciences, National Institutes for Quantum and Radiological Science and Technology, Chiba, Japan
- Department of Psychiatry, Nara Medical University, Kashihara, Japan
- Department of Neurology and Gerontology, Kyoto Prefectural University of Medicine, Kyoto, Japan
- Division of Neurology, Kobe University Graduate School of Medicine, Kobe, Japan
- Neurology Chiba Clinic, Chiba, Japan
- Department of Neurology, Graduate School of Medicine, Chiba University, Chiba, Japan
- Department of Clinical and Experimental Neuroimaging, Center for Development of Advanced Medicine for Dementia, National Center for Geriatrics and Gerontology, Obu, Japan
- Department of Radiopharmaceutics Development, National Institute of Radiological Sciences (NIRS), National Institutes for Quantum and Radiological Science and Technology (QST), Chiba, Japan
*:Corresponding author
Contact
Hitoshi Shimada (shimada.hitoshi=qst.go.jp) (replace"="with"@")
Department of Functional Brain Imaging Research,National Institute of Radiological Sciences, QST
