Tritium is one of hydrogen family (isotopes).
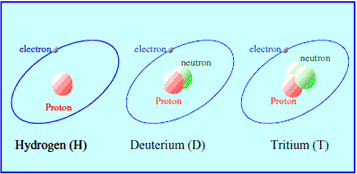
Tritium is one of thrce hydrogen isotopes, and its nucleus consists of a proton and two neutrons
Tritium is a radioactive material.
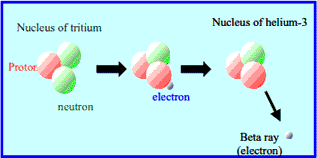
Tritium has radioactivity and decays to helium-3, a stable isotope of helium, by emitting beta rays (electrons) . It takes about 12.3 years (it is called " half life") that tritium decreases to half amount. The beta rays emitted from tritium can be shieled with a thin sheet due to its low energy.
Tritium in nature
Tritium is produced in nature by nuclear reactions between neutrons in cosmic radiation and nitrogen or oxygen in atmosphere. The amount of tritium produced by these processes is estimated to be 200g per year. 99% of tritium exists in nature in the form of tritiated water (HTO), like water vapor, rain and sea. So it circulates together with light water (H20) around our environment.
Tritium as a fuel of nuclear fusion
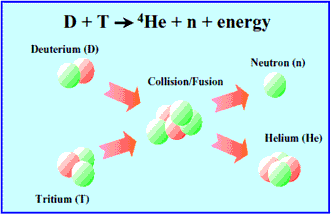
Nuclear fusion induced by both deuterium and tritium is considered to be the most promising reaction to realize fusion reactor. So tritium will be used as a fuel of nuclear fusion reactor in the future.
