Abstract
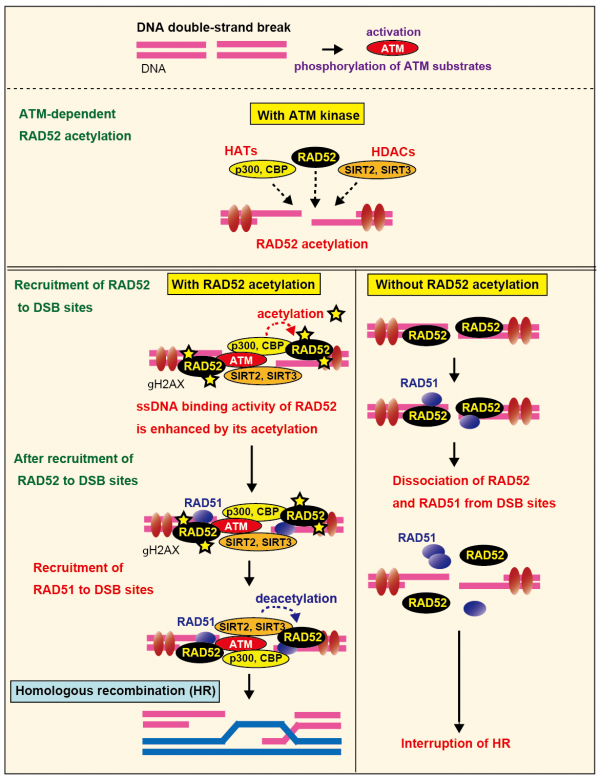
DNA double strand breaks (DSBs) are the most dangerous type of DNA damage in cells. Homologous recombination (HR) is a DSB repair system in which a central player, RAD51, functions with several proteins, including RAD52. DSBs activate the DNA damage response signaling network, in which the ataxia telangiectasia mutated (ATM) protein plays a chief role, by phosphorylating numerous target proteins. As compared to phosphorylated proteins, relatively few acetylated proteins have been functionally characterized in DNA repair. In addition, beyond the roles in phosphorylation signaling, much less is known about whether ATM functions are linked with other protein modifications, such as acetylation. Here, we found that RAD52 at DSB sites is acetylated by p300/CBP acetyltransferases and then deacetylated by SIRT2/SIRT3 deacetylases. RAD52 acetylation is required for sustained RAD51 colocalization at DSB sites, and is therefore essential in HR. ATM is required for the recruitment of RAD52, p300/CBP and SIRT2/SIRT3 to DSB sites, and therefore is essential for RAD52 acetylation. Thus, the RAD52-acetylation state is critical for HR, and its regulation is linked to ATM signaling. Our work demonstrates the importance of the regulation of RAD52 acetylation in HR and its underlying mechanism.
Image
Acetylation of human RAD52 is required for DSB repair by homologous recombination.
Reference
Yasuda T, Kagawa W, Ogi T, Kato TA, Suzuki T, Dohmae N, et al. (2018) Novel function of HATs and HDACs in homologous recombination through acetylation of human RAD52 at double-strand break sites. PLoS Genet 14(3): e1007277.
Novel function of HATs and HDACs in homologous recombination through acetylation of human RAD52 at double-strand break sites
Contact
Takeshi yasuda ( yasuda.takeshi=qst.go.jp ) ( replace "=" with "@" )
Department of Basic Medical Sciences for Raddiation Damages,National Institute of Radiological Sciences, QST
