Abstract
Mouse embryonic stem cells (ESCs) is pluripotent stem cells and differentiate into multiple cell types during organismal development. Fibroblast growth factor 4 (FGF4) signaling induces differentiation from ESCs via the phosphorylation of downstream molecules such as mitogen-activated protein kinase/extracellular signal-related kinase (MEK) and extracellular signal-related kinase 1/2 (ERK1/2). To maintain the undifferentiated state of ESCs, the FGF4-MEK-ERK1/2 pathway is inhibited. However, the inhibitory mechanism of the FGF4-MEK-ERK1/2 pathway in ESCs is uncharacterized.
O-linked β-N-acetylglucosaminylation (O-GlcNAcylation) is a post-translational modification characterized by the attachment of a single N-acetylglucosamine (GlcNAc) to the serine and threonine residues of nuclear or cytoplasmic proteins such as signal components, transcription factors, and epigenetic factors. O-GlcNAc modification competes with phosphorylation because OGT catalyzes the addition of O-GlcNAc at or in proximity to phosphorylation sites
Here, we showed that the O-GlcNAc on the phosphorylation site of PKCζ inhibits PKCζ phosphorylation (activation) and consequently, the FGF4-PKCζ-MEK-ERK1/2 pathway in ESCs. Our results demonstrate a novel mechanism for the maintenance of the undifferentiated state of ESCs via the inhibition of the FGF4-PKCζ-MEK-ERK1/2 pathway by O-GlcNAcylation on PKCζ.
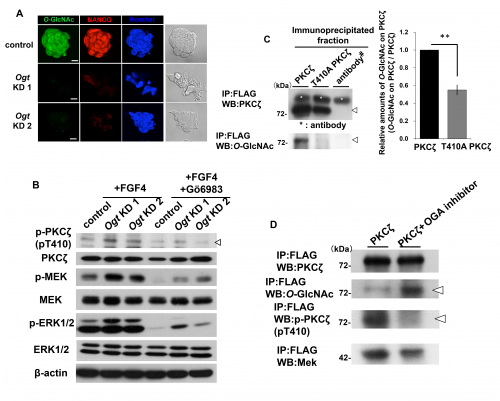
(A) Immunostaining using antibodies against O-GlcNAc, NANOG (marker of the undifferentiated state) in Ogt KD cells. Nuclei were stained with Hoechst (blue). Scale bars: 10 μm.
(B) Western blot analysis using antibodies against phospho-Thr-410 of PKCζ (p-PKCζ pT410), PKCζ, phospho-MEK (p-MEK), MEK, phospho-ERK1/2 (p-ERK1/2), and ERK1/2 in Ogt KD cells after FGF4 stimulation (50 ng/mL) for 3 min with or without 10 µM Gö6983 (PKC inhibitor).
(C) Western blot (WB) analysis using anti-PKCζ and anti-O-GlcNAc (RL-2) antibodies for the fraction immunoprecipitated (IP) with anti-FLAG antibody. Arrowheads indicate PKCζ (WB:PKCζ) and O-GlcNAc on PKCζ (WB:O-GlcNAc) co-immunoprecipitated with anti-FLAG antibody. The histograms show the mean densitometric readings ± SD of (O-GlcNAc on PKCζ co-immunoprecipitated with anti-FLAG antibody)/(PKCζ co-immunoprecipitated with anti-FLAG antibody)
after normalization against the levels in PKCζ-FLAG-expressing cells (set to 1). Values were obtained from three independent experiments, and significant values in comparison with cells expressing PKCζ-FLAG tag (Figure 5H) are indicated as follows: **, P < 0.01. #Only anti-FLAG antibody was loaded to detect the heavy and light chains of the antibodies used for immunoprecipitation. Asterisk (*) indicates the heavy chain or light chain of the anti-FLAG antibody used for immunoprecipitation.
(D) Western blot (WB) analysis using anti-PKCζ, anti-O-GlcNAc (RL-2), anti-phospho-Thr-410 of PKCζ (p-PKCζ pT410), and anti-MEK antibodies for the fraction immunoprecipitated (IP) with anti-FLAG antibody using cells expressing PKCζ-FLAG tag in the presence or absence of 200 μM PUGNAc (OGA inhibitor) for 24 h. Arrowheads indicate O-GlcNAc on PKCζ-FLAG (WB:O-GlcNAc) and the phosphorylation of Thr-410 of PKCζ-FLAG (WB:p-PKCζ pT410).
Reference
O-GlcNAc on PKCζ Inhibits the FGF4-PKCζ-MEK-ERK1/2 Pathway via Inhibition of PKCζ Phosphorylation in Mouse Embryonic Stem Cells
Taichi Miura,1,4 Masahiko Kume,2 Takeshi Kawamura,3 Kazuo Yamamoto,2 Takao Hamakubo,3
and Shoko Nishihara1,*
1Department of Bioinformatics, Graduate School of Engineering, Soka University
2Department of Integrated Biosciences, Graduate School of Frontier Sciences, The University of Tokyo
3Department of Molecular Biology and Medicine, Research Center for Advanced Science and Technology (RCAST), The University of Tokyo
4National Institute of Radiological Sciences (NIRS), National Institutes for Quantum and Radiological Science and Technology
*Correspondence
Contact
Taichi Miura (miura.taichi[at]qst.go.jp) *Note:Please change [at] into @.
Regenerative Therapy Research Team, National Institute of Radiological Sciences, QST
