Our daily use of radiation and nuclear energy relies on our knowledge of radiation safety. QST (and its predecessor NIRS) has therefore been conducting research on radiation safety since its establishment in 1957. Fundamental studies on health risks of radiation using experimental animals have been one of the fields where QST has been particularly strong.
As radiation use has become common in medical treatment of children such as examinations and therapies, we have been studying risk of radiation in children via animal experiments, where scientific data are insufficient. As Japan experienced two serious nuclear accidents (the JCO criticality accident in 1999 and the Fukushima Daiichi Nuclear Power Plant accident in 2011), we have been conducting scientific researches to respond to the wide concerns on the risk of low dose rate radiation.
How experimental researches contribute to understanding of radiation risks in human
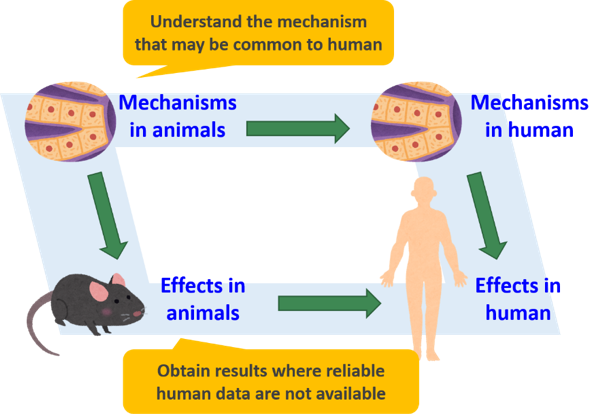
Radiation effects research must contribute to understanding of radiation effects in human (as one of animal species). But can results obtained in experimental animals play a role and, if so, how?
Observations in human are called epidemiology and they are valuable in that they directly observe radiation effects in human. However, epidemiology often accompanies difficulty in controlling study conditions, which include accurate estimation of radiation doses and the consistency in the lifestyle and other factors among people of the exposed and unexposed groups. Moreover, epidemiology is not applicable to situations that humans have never experienced. Experimental studies play essential roles in such situations, because they can place everything under control and simulate situations where corresponding epidemiological studies are unavailable.
In addition, experimental studies are powerful in obtaining essential insights into the mechanisms underlying our observations; if the mechanism is common to humans, we can reason that the same thing will be observed also in human. There, mathematical modeling will be especially important, as it will allow us to numerically predict what will happen in human.
Understanding radiation risks in children
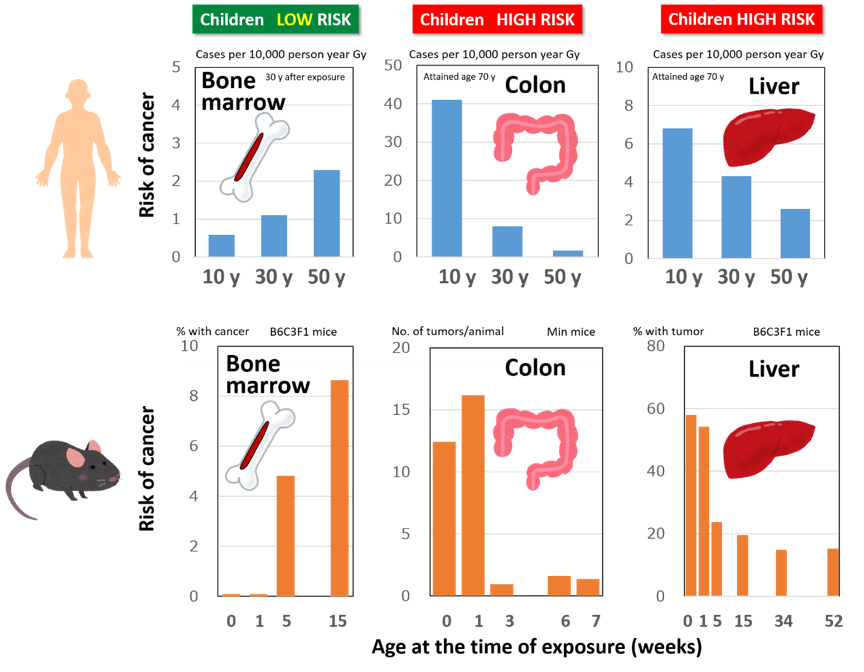
In the past decade, QST has been conducting biological studies using mice and rats to understand radiation effects in children, which are generally believed to be very sensitive to radiation, especially focusing on their future risk of cancer.
The first thing we confirmed is that the age dependence of sensitivity varies among tissues. Let’s take an example of myeloid leukemia (a type of cancer of the blood cells). Both epidemiology and animal studies tell us that radiation exposure as a child does not lead to higher risk of myeloid leukemia than exposure as an adult. It is thus opposite to our naïve understanding that children are more sensitive than adults. The risk of lung cancer exhibited a similar trend. On the contrary, risks of colon and liver cancers tended to be higher after children are exposed, which is consistent with the general image.
These examples tell us that children are more sensitive regarding one tissue and adults are sensitive in another. But what is behind such difference among tissues?
Cancer is caused by errors in important genes in the cells. Radiation may damage the genes, but cells will not become cancerous if the cells are removed. Risk of cancer increases only if cells with erroneous genes persist and increase in the body. This reasoning led us to studies that examine the removal and persistence of cells in individual tissues and compare them between young and adult animals.
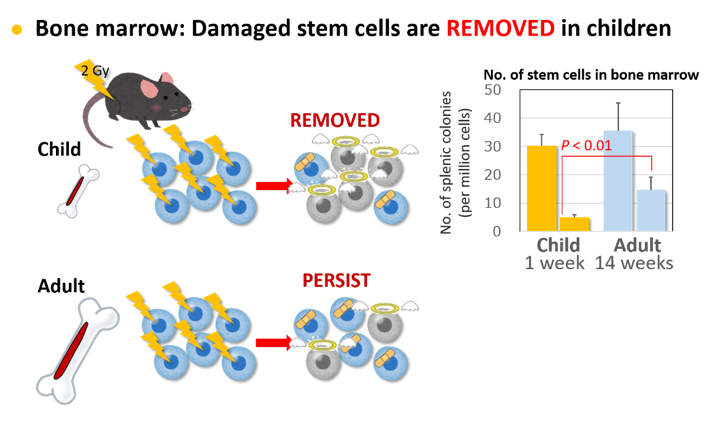
Bone marrow has a pool of cells (called stem and progenitor cells) that are essential for producing new blood cells. Exposure of young mice to radiation resulted in a marked decrease in these cells, whereas exposure of adult mice allowed several times more cells to survive and persist. As cancer may arise from cells that persist, this finding explains why exposure as adults leads to higher risk of myeloid leukemia than exposure as children.
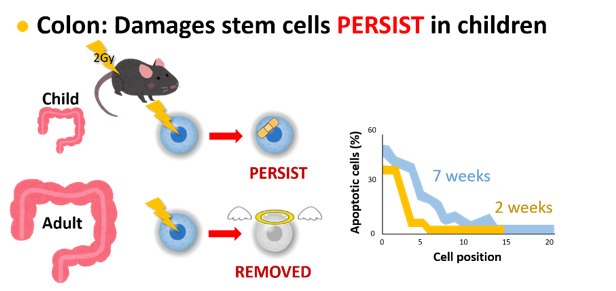
Regarding the colon, we found an opposite situation where stem and progenitor cells of young mice tend to survive after radiation exposure whereas those cells of adults are more sensitive to induction of cell death. In the liver of young mice, cells are undergoing rapid cell divisions to increase the cell population, and radiation exposure further increased the cell divisions, while cells of adults are resting regardless of radiation exposure. These observations explain why exposure as children leads to higher risk of colon and lung cancers than exposure as adults.
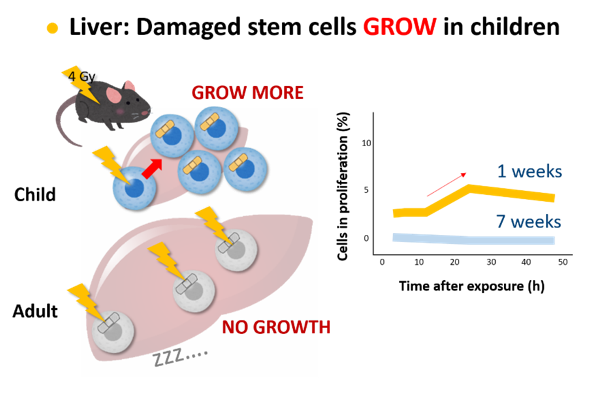
Textbook of radiation biology says that stem cells in the colon undergo rapid cell death after radiation exposure, and that cells exposed radiation stop their process of cell division. Such textbook knowledge is not applicable to children!
These results present underlying mechanisms of age-dependent radiation risks observed in epidemiology and animal experiments, telling us that the tissue-to-tissue difference in the age difference is not an artefact but an outcome of the biological properties of individual tissues.
References
- Sasaki. Influence of the age of mice at exposure to radiation on life-shortening and carcinogenesis. J Radiat Res 32 Suppl 2:73-85 (1991)
- Miyoshi-Imamura et al. Unique characteristics of radiation-induced apoptosis in the postnatally developing small intestine and colon of mice. Radiat Res 173(3):310-318 (2010)
- Ariyoshi et al. Age dependence of hematopoietic progenitor survival and chemokine family gene induction after gamma irradiation in bone marrow tissue in C3H/He mice. Radiat Res 181(3):302-313 (2014)
- Shang et al. Radiation Exposure Enhances Hepatocyte Proliferation in Neonatal Mice but not in Adult Mice. Radiat Res 188(2):235-241 (2017)
A claw mark of radiation left in the genome of lymphoma

It is believed that one mechanism by which radiation increases the risk of cancer is through its ability to damage DNA (or more precisely, induce chemical changes such as oxidation and breaks). Especially, once the double helix of the DNA molecule is cut (or DNA a double strand break is induced), cells are often able to repair it only imperfectly, leading to an unintended change in the genetic information embedded in the molecule, a mutation. And once this happens in one of the important genes regulating cellular proliferation and differentiation, a cell harboring the mutation will remain in the body and may cause cancer in the future.
If this is real, it is assumed that cells of a radiation-induced cancer should have a mutation which is a trace of an important genes being once severed and incorrectly reconnected—so to speak a “claw mark of radiation”.

Previously, such a claw mark was pursued in lung cancers of miners who had worked in environments with high concentration of radon (a radiation-emitting gas) in air. They studied a gene called p53, which can be a cause of cancer if mutated, in those lung cancers but did not find anything like a “claw mark of radiation” there.
In our study, we focused on thymic lymphoma (a type of blood cell cancer like leukemia in human) that frequently develops in mice exposed to repeated high doses of radiation. Large-scale changes are expected after radiation makes many DNA strand breaks and the cell reconnects them in an incorrect manner. So, we used a hybrid strain of two types of mice (B6 and C3H strains) and exposed these hybrid mice to radiation to induce thymic lymphoma. We searched for DNA regions in which a trait of either strain (B6 or C3H) was lost. This approach has been often used to locate causative mutations in cancer genome. While normal cells have traits of both strains over the whole genome, cancer cells might have places where one of them is lacking (which is called loss of heterozygosity).
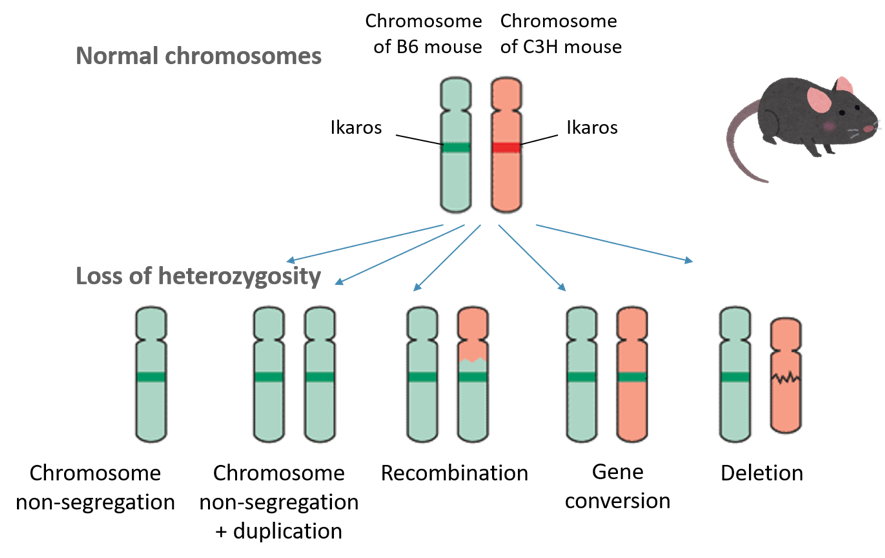
The result indicated a frequent loss of heterozygosity in a region containing the gene Ikaros in radiation-induced thymic lymphomas. This was not the case with lymphomas induced by a chemical substance that does not induced DNA breaks. The gene Ikaros is essential for the maturation of T cell in thymus, an organ where thymic lymophoma develops. So, if Ikaros does not work properly, T cells will stay immature and proliferative. The story may be like this: radiation first severed a DNA strand near the gene Ikaros in an immature T cell, the cell subsequently reconnected the DNA end with an incorrect end, losing one of the two Ikaros genes that had been inherited from B6 and C3H mice, and thus an immature cell remained in the thymus. The loss of heterozygosity spanning Ikaros is a claw mark of radiation!

This study proves the validity of the idea that incorrect repairing of radiation-induced DNA strand breaks leads to mutation of important genes and finally to cancer, at least in the case of thymic lymphoma of mice.
References
- Shimada et al. Radiation-associated loss of heterozygosity at the Znfn1a1 (Ikaros) locus on chromosome 11 in murine thymic lymphomas. Radiat Res 154(3):293-300 (2000)
- Kakinuma et al. Spectrum of Znfn1a1 (Ikaros) inactivation and its association with loss of heterozygosity in radiogenic T-cell lymphomas in susceptible B6C3F1 mice. Radiat Res 157(3):331-340 (2002)
- Yoshida et al. Distinct structural abnormalities of chromosomes 11 and 12 associated with loss of heterozygosity in X-ray-induced mouse thymic lymphomas. Cancer Genet Cytogenet 179(1):1-10 (2007)
- Sunaoshi et al. The effect of age at exposure on the inactivating mechanisms and relative contributions of key tumor suppressor genes in radiation-induced mouse T-cell lymphomas. Mutat Res 779:58-67 (2015)
Press releases and news
Please see other results of our studies published below (Japanese only).
- Acceleration of cancer mortality risk due to radiation exposure differs about 100-fold between humans and mice: a mathematical analysis of the relationship between aging, cancer development, and radiation exposure (May 1, 2024)
- Azithromycin, a drug that "reads-through" genetic mutations, prevents cancer. Mouse experiment shows the preventive effect against hereditary colorectal cancer (June 23, 2023)
- A new animal model of hereditary breast cancer. Expectation for elucidating the mechanisms of cancer risk and prevention (August 22, 2022)
- An accurate method established for evaluating the power of neutron radiation to induce cancer. A new way to obtain data as the basis for radiation protection global standards (July 24, 2021)
- Improving diet may reduce colorectal cancer after irradiation as an experiment shows with mice on calorie restriction (March 5, 2021)
- How radiation induces cancer not via direct generation of mutations. The whole picture of genetic mutations in a mouse model of hereditary lymphoma (March 25, 2019)
- Cancer risk of “slow” radiation exposure differs between child and adult. Rat mammary cancer decreases more in adults when dose rate is reduced (February 4, 2019)
- Risk of breast cancer after radiation exposure reduced by experience of pregnancy and delivery. Its mechanism revealed by a rat experiment, paving a way for a drug that reduces breast cancer risk (November 15, 2018)
- A new mechanism by which radiation induces breast cancer. Expectations on risk evaluation and development of prevention method(May 25, 2018)
- Cancer-inducing effect of “slow” radiation exposure revealed by an animal experiment. Direct measurement of cancer risk from low dose rate radiation exposure in an animal model (December 13, 2016)
- New experiment revealing safety of carbon ion radiation therapy. Children may have relatively small risk of breast cancer in the future (October 23, 2012)

How our research contributing to radiation safety and regulations

Many aspects of our daily life rely on good use of radiation. On the other hand, wrong use of radiation may affect human health. It is therefore important that safety of radiation use is validated by state-of-the-art science. Our fundamental research produces such state-of-the-art understanding, contributing to safe use of radiation.
Domestic regulations and medical guidelines on radiation use follow recommendations and standards issued by radiation safety-related international organizations such as International Commission on Radiological Protection (ICRP) and International Atomic Energy Agency (IAEA). The recommendations and standards are based on scientific knowledge of effects of radiation, which is reviewed by organizations such as United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR). Our scientific papers contribute to reports by such organizations as their source of scientific information.
Recent citations of our research in important reports
- Cited in UNSCEAR White Paper “Biological Mechanisms of Radiation Actions at Low Doses” (2012)
- Imaoka et al. J Radiat Res 49(4): 349-360 (2008)
- Vares, Wang, Shang et al. Int J Radiat Biol 85(1): 70-86 (2009)
- Cited in National Council of Radiation Protection and Measurements (NCRP) Report No. 171 “Uncertainties in the Estimation of Radiation Risks and Probability of Disease Causation” (2012)
- Yamauchi, Kakinuma, Shimada et al. (2008) Mutat Res 640(1-2), 27-37
- Yamauchi, Kakinuma, Shimada et al. (2008) Mutat Res 640(1-2), 27-37
- Cited in International Agency for Research on Cancer (IARC) Monograph 100D “A Review of Human Carcinogens: Radiation” (2012)
- Imaoka, Nishimura, et al. Int J Radiat Oncol Biol Phys 69:194-203, 2007
- Imaoka, Okamoto, et al. Radiat Res 165:165-173, 2006
- Cited in ICRP Publication 127 “Radiological Protection in Ion Beam Radiotherapy” (2014)
- Imaoka, Nishimura, et al. Int J Radiat Oncol Biol Phys 69(1):194-203, 2007
- Cited in ICRP Publication 131 “Stem Cell Biology with Respect to Carcinogenesis Aspects of Radiological Protection” (2015)
- Shimada et al. Radiat Res 137(1):118-123, 1994
- Imaoka, Nishimura, et al. Int J Radiat Oncol Biol Phys 69:194-203, 2007
- Imaoka, Nishimura, et al. Mol Carcinog 50(7):539-552, 2011
- Cited in NCRP Commentary No. 24 “Health Effects of Low Doses of Radiation: Perspectives on Integrating Radiation Biology and Epidemiology” (2015)
- Nakano et al. Radiat Res 181(2):172-176 (2014)






