
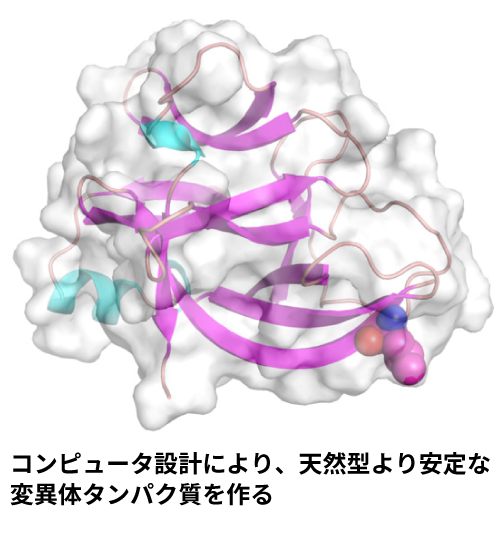
 生体分子シミュレーションチームでは計算科学的アプローチと実験科学的アプローチの双方を用い、生体高分子の運動を理解し、新たな機能を創成する研究を行っています。研究には計算科学的アプローチとして分子モデリング・構造予測、分子シミュレーション、構造生成AIなどを利用しつつ、実験科学的アプローチとして光ピンセットを用いた単一分子計測や遺伝子工学を活用しています。計算・実験の組み合わせによりタンパク質や核酸複合体などの生体高分子の動きと機能発揮のメカニズムを解き明かし、そこに低分子薬剤・アミノ酸変異・タンパク質設計など様々な手法で介入をすることが研究の目的です。特に、低分子とタンパク質との結合・解離、DNA収納状態の変化と遺伝子発現の関係、機能性タンパク質の創製研究を行っています。
生体分子シミュレーションチームでは計算科学的アプローチと実験科学的アプローチの双方を用い、生体高分子の運動を理解し、新たな機能を創成する研究を行っています。研究には計算科学的アプローチとして分子モデリング・構造予測、分子シミュレーション、構造生成AIなどを利用しつつ、実験科学的アプローチとして光ピンセットを用いた単一分子計測や遺伝子工学を活用しています。計算・実験の組み合わせによりタンパク質や核酸複合体などの生体高分子の動きと機能発揮のメカニズムを解き明かし、そこに低分子薬剤・アミノ酸変異・タンパク質設計など様々な手法で介入をすることが研究の目的です。特に、低分子とタンパク質との結合・解離、DNA収納状態の変化と遺伝子発現の関係、機能性タンパク質の創製研究を行っています。
当研究チームでは随時研究者を募集しています。興味のある方はチームリーダーまで相談ください。

チームリーダー 櫻庭 俊
メンバー
 櫻庭 俊 チームリーダー
櫻庭 俊 チームリーダー
石田 恒 上席研究員
河野 秀俊 上席研究員
松本 淳 主幹研究員
角南 智子 主幹研究員
小野 純一 主任研究員
Kumar Amarjeet 研究員
Chan Wai Soon (Justin) 研究員
原 祐子 研究補助員
原澤伸治 研究補助員
市村 智明 学生実習生
主な論文成果
- Sunami T, Luo D, Sato S, Kato J, Yamanaka M, Akamatsu K, Kurumizaka H, Kono H. FRET analysis of the unwrapping of nucleosomal DNA containing a sequence characteristic of the +1 nucleosome. Scientific Reports. 2025 15:2169 doi:10.1038/s41598-025-86075-y.
- Asama R, Tominaga M, Ito S, Ito Y, Takemura K, Sakuraba S, Katsurada K, Fukuda N, Kondo A, Ishii J. Screening of protein-based inhibitors for the intracellular domain of epidermal growth factor receptor by directed evolution using the yeast Gγ recruitment system. Journal of bioscience and bioengineering 2024 138:375.
- Taguchi M, Sakuraba S, Chan J, Kono H. Unveiling the Photoactivation Mechanism of BLUF Photoreceptor Protein through Hybrid Quantum Mechanics/Molecular Mechanics Free-Energy Calculation. ACS Physical Chemistry Au 2024 4(6):647 doi: 10.1021/acsphyschemau.4c00040.
- Watanabe K, Yamamoto T, Fujita T, Hino S, Hino Y, Yamazaki K, Ohashi Y, Sakuraba S, Kono H, Nakao M, Ochiai K. Metabolically inducing defects in DNA repair sensitizes BRCA–wild-type cancer cells to replication stress. Science Signaling 2024, 17:eadl6445 doi:10.1126/scisignal.adl6445.
- Ishida H, Matsumoto A, Tanaka H, Okuda A, Morishima K, Paul AW, Kurumizaka H, Sugiyama M, Kono H: Structural and Dynamic Changes of Nucleosome upon GATA3 binding. Journal of Molecular Biology 2023, 435:168308. doi:10.1016/j.jmb.2023.168308.
- Fujimura A, Ishida H, Nozaki T, Terada S, Azumaya Y, Ishiguro T, Kamimura Y, Kujirai T, Kurumizaka H, Kono H, et al.: Designer adaptor proteins for functional conversion of peptides to small-molecule ligands toward in-cell catalytic protein modification. ACS Central Science 2023, 9:2115–2128 doi:10.1021/acscentsci.3c00930.
- Chan-Yao-Chong M, Chan W, Soon, Kono H: Benchmarking of force fields to characterize the intrinsically disordered R2‑FUS‑LC region. Scientific Reports 2023, 13:14226 doi:10.1038/s41598-023-40801-6.
- Yunoki Y, Matsumoto A, Morishima K, Martel A, Porcar L, Sato N, Yogo R, Tominaga T, Inoue R, Yagi-Utsumi M, et al.: Overall structure of fully assembled cyanobacterial KaiABC circadian clock complex by an integrated experimental-computational approach. Commun Biol 2022, 5:184 doi:10.1038/s42003-022-03143-z.
- Higo J, Kasahara K, Bekker G-J, Ma B, Sakuraba S, Iida S, Kamiya N, Fukuda I, Kono H, Fukunishi Y, et al.: Fly casting with ligand sliding and orientational selection supporting complex formation of a GPCR and a middle sized flexible molecule Scientific Reports 2022, 12:13792 doi:10.1038/s41598-022-17920-7.
- Ishida H, Kono H: Free Energy Landscape of H2A-H2B Displacement From Nucleosome. J Mol Biol 2022, 434:167707 doi:10.1016/j.jmb.2022.167707.
- Chan J, Kumar A, Kono H: RNAPII driven post-translational modifications of nucleosomal histones. Trends in Genetics 2022, 38:1076-1095 doi:10.1016/j.tig.2022.04.010.
- Sakuraba S, Xie Q, Kasahara K, Iwakiri J, Kono H: Extended ensemble simulations of a SARS-CoV-2 nsp1-5'-UTR complex. PLoS Comput Biol 2022, 18:e1009804 doi:10.1371/journal.pcbi.1009804.
- Sato K, Kumar A, Hamada K, Okada C, Oguni A, Machiyama A, Sakuraba S, Nishizawa T, Nureki O, Kono H, et al.: Structural basis of the regulation of the normal and oncogenic methylation of nucleosomal histone H3 Lys36 by NSD2. Nat Commun 2021, 12:6605 doi:10.1038/s41467-021-26913-5.
- Ohtomo H, Kurita JI, Sakuraba S, Li Z, Arimura Y, Wakamori M, Tsunaka Y, Umehara T, Kurumizaka H, Kono H, et al.: The N-terminal Tails of Histones H2A and H2B Adopt Two Distinct Conformations in the Nucleosome with Contact and Reduced Contact to DNA. J Mol Biol 2021, 433:167110 doi:10.1016/j.jmb.2021.167110.
- Kumar A, Chan J, Taguchi M, Kono H: Interplay among transacting factors around promoter in the initial phases of transcription. Curr Opin Struct Biol 2021, 71:7-15 doi:10.1016/j.sbi.2021.04.008.
- Ishida H, Kono H: Torsional stress can regulate the unwrapping of two outer half superhelical turns of nucleosomal DNA. Proc Natl Acad Sci USA 2021, 118:e2020452118 doi:10.1073/pnas.2020452118.
- Sunami T, Hirano Y, Tamada T, Kono H: Structural basis for an array of engrailed homeodomains. Acta Crystallographica Section D 2020, 76:824-833 doi:10.1107/S2059798320009237.
- Kono H, Ishida H: Nucleosome unwrapping and unstacking. Curr. Opin. Struct. Biol. 2020, 64:119-125 doi:doi.org/10.1016/j.sbi.2020.06.020.
- Matsumoto A, Sugiyama M, Li Z, Martel A, Porcar L, Inoue R, Kato D, Osakabe A, Kurumizaka H, Kono H: Structural Studies of Overlapping Dinucleosomes in Solution. Biophys J 2020, 118:2209-2219 doi:10.1016/j.bpj.2019.12.010.
- Takehara S, Sakuraba S, Mikami B, Yoshida H, Yoshimura H, Itoh A, Endo M, Watanabe N, Nagae T, Matsuoka M, et al.: A common allosteric mechanism regulates homeostatic inactivation of auxin and gibberellin. Nature Communications 2020, 11:2143 doi:10.1038/s41467-020-16068-0.
- Murakawa T, Kurihara K, Shoji M, Shibazaki C, Sunami T, Tamada T, Yano N, Yamada T, Kusaka K, Suzuki M, et al.: Neutron crystallography of copper amine oxidase reveals keto/enolate interconversion of the quinone cofactor and unusual proton sharing. Proceedings of the National Academy of Sciences 2020, 117:10818-10824 doi:10.1073/pnas.1922538117.
- Takahashi H, Sakuraba S, Morita A: Large-Scale Parallel Implementation of Hartree-Fock Exchange Energy on the Real-Space Grids Using 3D-Parallel Fast Fourier Transform. Journal of Chemical Theory and Computation 2020, 60:1376 -1389 doi:10.1021/acs.jcim.9b01063.
- Kumar A, Kono H: Heterochromatin protein 1 (HP1): interactions with itself and chromatin components. Biophys Rev. 2020, 12:387-400 doi:10.1007/s12551-020-00663-y.
- Klein BJ, Jang SM, Lachance C, Mi W, Sakuraba S, Krajewski K, Lyu J, Wang WW, Sidoli S, Yan K, et al.: Histone H3K23-specific acetylation by MORF is coupled to H3K14 acylation. Nature Comm. 2019, 10:4724 doi:10.1038/s41467-019-12551-5.
- Sunami T, Kono H: Balance between DNA-binding affinity and specificity enables selective recognition of longer target sequences in vivo. Protein Science 2019, 28:1630-1639 doi:10.1002/pro.3677.
- Takahashi H, Suzuoka D, Sakuraba S, Morita A: Role of the photosystem II as an environment on the oxidation free energy of the Mn cluster from S1 to S2. J. Phys. Chem. B. 2019, 123:7081-7091 doi:10.1021/acs.jpcb.9b03831.
- Li Z, Kono H: Investigating the Influence of Arginine Dimethylation on Nucleosome Dynamics Using All-Atom Simulations and Kinetic Analysis. The Journal of Physical Chemistry B 2018, 122:9625-9634 doi:10.1021/acs.jpcb.8b05067.
- Luo D, Kato D, Nogami J, Ohkawa Y, Kurumizaka H, Kono H: MNase, as a probe to study the sequence-dependent site exposures in the +1 nucleosomes of yeast. Nucleic Acids Research 2018, 46:7124-7137 doi:10.1093/nar/gky502.
- Kono H, Sakuraba S, Ishida H: Free Energy Profiles for Unwrapping the Outer Superhelical Turn of Nucleosomal DNA. PLoS Comp. Biol. 2018, 14:e1006024 doi:10.1371/journal.pcbi.1006024.
- Gatchalian J, Wang X, Ikebe J, Luo D, Gibsono M, Zhang Y, Cox K, Musselman CA, Poirior MG, Kono H, et al.: Accessibility of the histone H3 tail in the nucleosome for binding of paired readers. Nat. Comm. 2017, 8:1489 doi:10.1038/s41467-017-01598-x.
- Tencer AH, Cox KL, Di L, Bridgers JB, Lyu J, Wang X, Sims JK, Tyler, Allen HF, Zhang Y, et al.: Covalent modifications of histone H3K9 promote binding of CHD3. Cell Reports 2017, 21:455-466 doi:10.1016/j.celrep.2017.09.054.
- Ishida H, Kono H: H4 tails potentially produce the diversity in the orientation of two nucleosomes. Biophysical J. 2017, 113:978-990 doi:10.1016/j.bpj.2017.07.015.
- Kato D, Osakabe A, Mizukami Y, Adachi F, Arimura Y, Saikusa K, Akashi S, Nishimura Y, Park S-Y, Matsumoto A, et al.: Crystal structure of the overlapping dinucleosome composed of hexasome and octasome. Science 2017, 356:205-208 doi:10.1126/science.aak9867.
- Ikebe J, Sakuraba S, Kono H: H3 Histone Tail Conformation within the Nucleosome and the Impact of K14 Acetylation Studied Using Enhanced Sampling Simulation. PLoS Comput Biol 2016, 12:e1004788 doi:10.1371/journal.pcbi.1004788.
- Sakuraba S, Kono H: Spotting the difference in molecular dynamics simulations of biomolecules. J. Chem. Phys. 2016, 145:074116 doi:10.1063/1.4961227.
- Ishida H, Matsumoto A: Mechanism for verification of mismatched and homoduplex DNAs by nucleotides-bound MutS analyzed by molecular dynamics simulations. Proteins 2016, 84:1287-1303 doi:10.1002/prot.25077.
- Ikebe J, Umezawa K, Higo J: Enhanced sampling simulations to construct free-energy landscape of protein–partner substrate interaction. Biophysical Reviews 2015, 10.1007/s12551-015-0189-z:1-18 doi:10.1007/s12551-015-0189-z.
- Tsukasaki Y, Miyazaki N, Matsumoto A, Nagae S, Yonemura S, Tanoue T, Iwasaki K, Takeichi M: Giant cadherins Fat and Dachsous self-bend to organize properly spaced intercellular junctions. Proc Natl Acad Sci U S A 2014, 111:16011-16016 doi:10.1073/pnas.1418990111.
- Yang L, kitano A, Huang B, Go N: Ligand-induced protein responses and mechanical signal propagation described by linear response theories. Biophysical Journal 2014, 107:1415-1425 doi:10.1016/j.bpj.2014.07.049.
Contact
量子生命科学研究所 量子生命システムグループ 生体分子シミュレーションチーム
所在地:QST千葉地区 〒263-8555 千葉市稲毛区穴川4-9-1
櫻庭 俊 チームリーダー sakuraba.shun[=]qst.go.jp ([=]を@にしてください)
